Structure and Reactivity Group
Centro de Química e Bioquímica
Departamento de Química e Bioquímica
Research
From structure to properties...
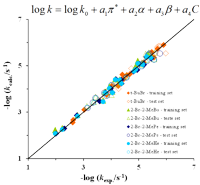 Accurate experimental data is used to rationalize solute (or substrate)-solvent-solvent interactions in reactivity and in equilibrium processes in solution and liquid interfaces. This field of research has been central to SRG and much progress has been achieved, for instance, in the understanding of molecular behaviors in solution through a meticulous application of quantitative structure-property relationships (QSPR).
The integrated use of various statistical and machine learning techniques (MLT),
Accurate experimental data is used to rationalize solute (or substrate)-solvent-solvent interactions in reactivity and in equilibrium processes in solution and liquid interfaces. This field of research has been central to SRG and much progress has been achieved, for instance, in the understanding of molecular behaviors in solution through a meticulous application of quantitative structure-property relationships (QSPR).
The integrated use of various statistical and machine learning techniques (MLT),
 namely, multiple linear regressions (MLR), neural networks (NN) and random forests (RF), as well as the concomitant use of stringent internal and external validation methodologies, has allowed the building of robust and predictive QSPRs in very different areas such as the modeling of solvent effects on rate constants, solution enthalpies and transition energies; the design of new antitubercular compounds (link); the evaluation of printing papers’ performance; and the assessment of pollutants’ extraction mechanisms (link), among others.
namely, multiple linear regressions (MLR), neural networks (NN) and random forests (RF), as well as the concomitant use of stringent internal and external validation methodologies, has allowed the building of robust and predictive QSPRs in very different areas such as the modeling of solvent effects on rate constants, solution enthalpies and transition energies; the design of new antitubercular compounds (link); the evaluation of printing papers’ performance; and the assessment of pollutants’ extraction mechanisms (link), among others.
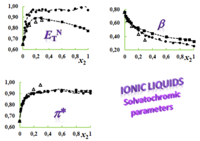
Systems studied thus far comprise haloalkanes, adamantyl, quinoline and carbamate derivatives; hydroxylic compounds; crown ethers; (new) solvatochromic probes; imidazolium-based ILs; isoniazid, benzimidazole, thiobenzanilides, indole and cinnamic acid derivatives; BXTEs and phenolic compounds.
Solvent engineering is a fairly new concept which includes the design and fine-tuning of new “solvents” to totally or partially replace commonly used solvents with impaired physicochemical properties or causing serious environmental and/or health problems.
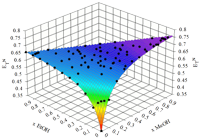
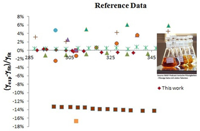
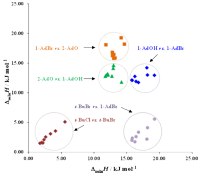
The expertise of SRG members in terms of the evaluation of reliable physicochemical properties such as solvatochromic parameters, densities, ultrasound speeds, refractive indices, surface tensions, electrical conductivities, solution enthalpies, and derived quantities, is of fundamental importance for the complete physicochemical and structural characterization of conventional and non-conventional alternative solvent mixtures, including ILs, the assessment of preferential solvation and mixing and excess behaviors, and even the proposal of reference data (Project PTDC/QUI/66826/2006, in collaboration with CCMM, ITQB and CQE). Some of these solvents or mixtures may eventually be used in the future for the development of greener solvent media for synthetic and/or separation technologies (link).
Systems studied up to now span from pure solvents to binary and ternary mixtures of protic/aprotic solvents such as alcohols with acetonitrile and/or formamide; mixtures of alkoxylamines or alkoxyalcohols with water (or alcohols); alkylammonium-based ILs, and their mixtures with alcohols; imidazolium- and dicyanamide-based ILs.
In the quest of new drugs...
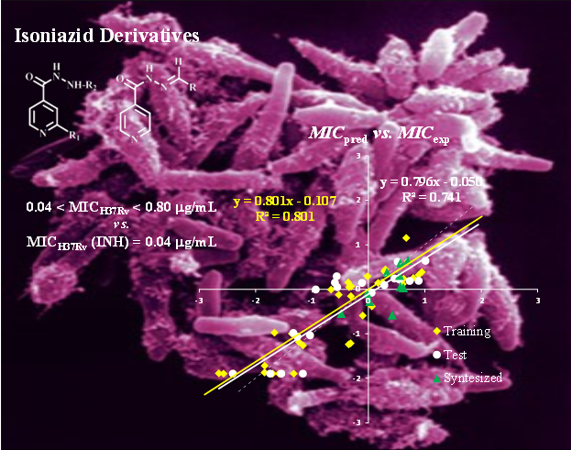
Tuberculosis (TB) is the second cause of death from a single infectious agent, the Mycobacterium tuberculosis bacillus. Nearly two billion people are currently infected with TB. In 2011 the World Health Organization (WHO) reported 8.7 million new TB cases and 1.4 million deaths. The emergence of multidrug-resistant (MDR-TB) and extensively drug-resistant TB (XDR-TB) dramatically reduces the number of available drugs for treatment, making the quest for new and efficient drugs a major public health concern. The establishment of quantitative relationships between structural features and biological activities is one of the most effective ways to design and develop new bioactive molecules.
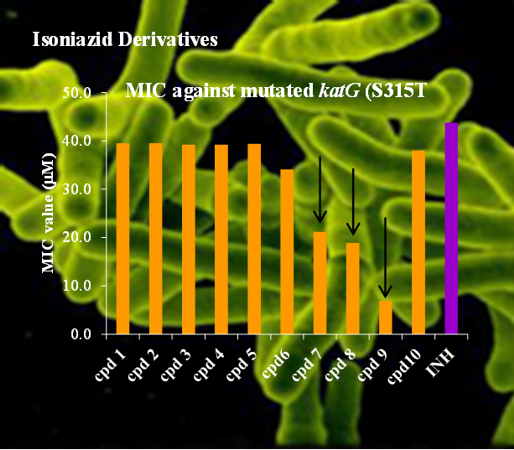
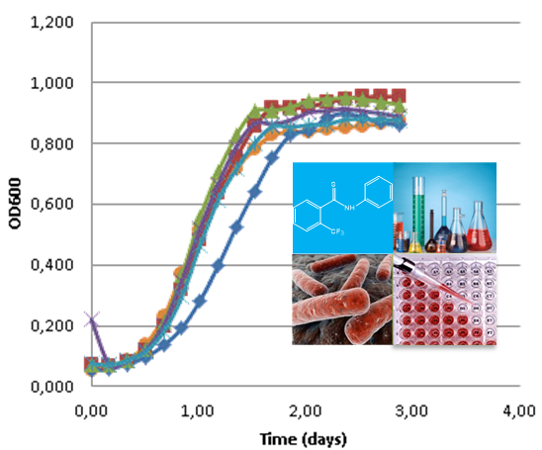
In the scope of two financed projects (FCT, POCTI/FCB/39709/2001 and FCT, PTDC/QUI/67933/2006, the latter in collaboration with FCT-UNL, FFUL and IHMT-UNL), SRG has been addressing the issue of rationally developing new potential antitubercular compounds by using various QSAR methodologies (MLR, NN, RF) supported by rigorous internal and external validation procedures. A systematic study carried out with four chemical families, namely isoniazid (INH), benzimidazole, thiobenzanilide and indole derivatives, with reported anti-TB activity, has allowed the building of several robust and predictive QSAR models that permitted the design, synthesis and characterization of 32 new potentially active anti-TB compounds. In particular, the in vitro evaluation of the activity of the synthesized INH derivatives against both wild type and INH mono-resistant Mycobacterium tuberculosis strains showed that 5 of these compounds exhibited higher activities than the reference compound (INH) and one presented a six fold increase in activity against the mutated katG (S315T) strain, by comparison with INH. This finding is especially relevant taking in consideration that INH is still the treatment of choice for TB, and new INH-based compounds showing activity against drug resistant tuberculosis will certainly be more effective, less expensive and less problematical than totally new compounds with unknown adverse effects such as those used in combination regimens currently recommended for TB.
Future work will involve a “hit-to-lead” optimization of selected compounds through chemical modification of hit structures, suggested by structure (or ligand)-based design. On going Molecular Dynamics studies (in collaboration with the Inorganic Theoretical Chemistry Group of CQB) and evaluation of experimental binding constants (along with University of Barcelona) will be used to rationalize differences in activity in susceptible and resistant strains.
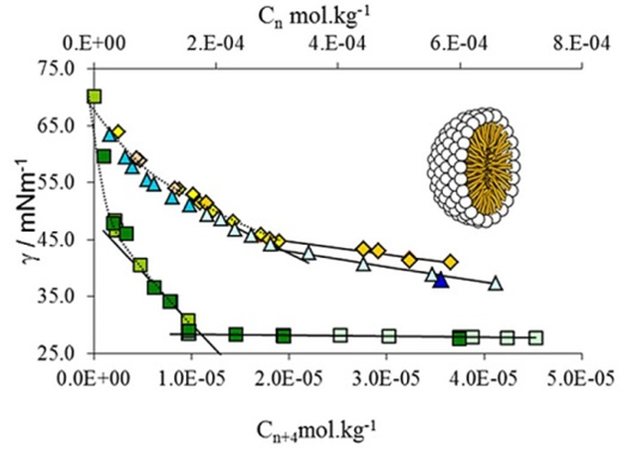
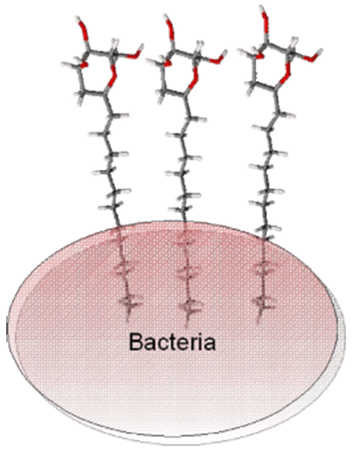
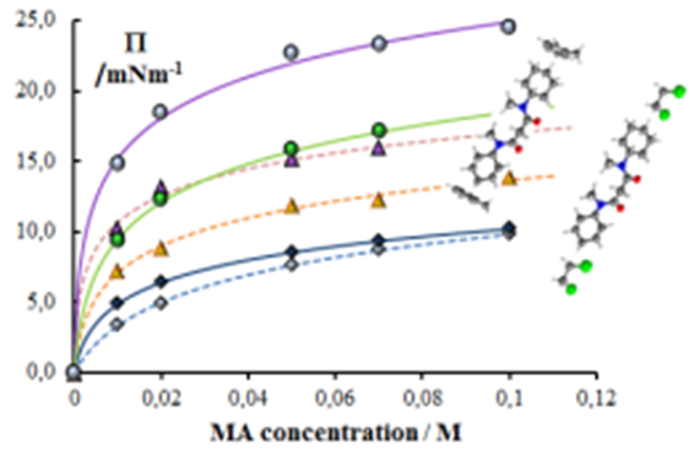
SRG is also engaged in the evaluation of partition/distribution equilibria on liquid interfaces, as well as on pKa and lipophilicity determination, given the importance of these processes/properties in the preliminary screening of new potentially active molecules. Within this framework, the surface activity and aggregation of novel amphiphilic glycoside derivatives have been evaluated to screen for new lead compounds with improved antimicrobial activity (QREN-SI I&DT Co-Promotion-Project nº 21547, in collaboration with the Carbohydrate Chemistry Group (CCG) of CQB).
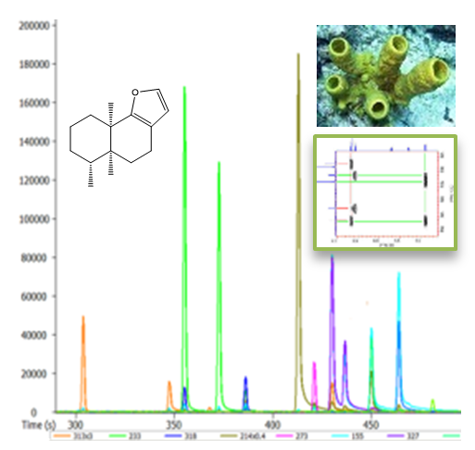
Natural products (NP) have been over human history a matchless source of drugs. Despite the competition from other drug discovery methods, NPs are still currently in the forefront of the search for novel leads. Between 2005 and 2010 nineteen NP based drugs were approved for marketing worldwide, among which seven have been classified as NPs, ten as semi-synthetic NPs, and two as NP derived drugs. Compounds from marine origin (sponges, microorganisms, etc.) are less explored than their terrestrial counterparts mainly due supply related issues. However, their unique structures and mechanisms of action make them an invaluable source of new drug leads, and research in this area has already provided seven approved drugs, the majority of them in the area of cancer.
Although half of the Portuguese borders are coastal areas, spanning through more than 700 miles, the research on marine organisms has been very scarce.
Harbored in two financed projects (FCT, PTDC/QUI-QUI/098053/2008 and QREN-SI I&DT Co-promotion - Project nº 13107) SRG has been developing work in this area, in collaboration with ITCG/CQB, aiming at:
- the bioassay guided isolation and structure characterization of secondary metabolites from marine invertebrates and their microbiota (particularly sponges from Erylus family); and
- the biological evaluation of the isolated compounds against targets involved in cancer and CNS disorders, e.g., IDO, TAU and TTR proteins.
Towards a cleaner environment
VOCs replacement in industrial processes encompasses a deep knowledge of specific physicochemical properties of alternative new solvents (link).
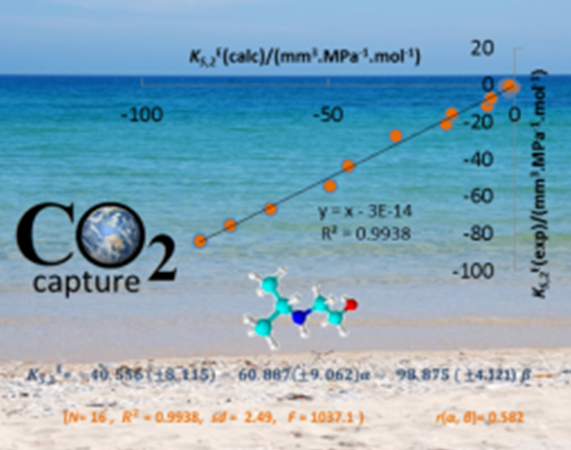
Amines, in particular aqueous amino alcohols and alkoxyamine solutions, are widely used for the removal of acid gases from a variety of gas streams of oil and natural gas industries being potential target solvents for CO2 trapping. The choice of the appropriate amine-based system for CO2 capture depends on the selectivity for CO2 rather than for other acid gases such as H2S, on a low corrosiveness in the presence of oxygen and other impurities, and, very significantly, on a favourable kinetic and energetic balance between CO2 capture and amine regeneration for recycling. Again, a complete thermodynamic and physicochemical knowledge and characterization of the chosen amine solvent systems is absolutely crucial to optimize the various stages of CO2 removal, since there seems to be a structure-performance relationship between this new generation of solvents and CO2 capture (link). Interestingly, these solvents may behave as Switchable-Polarity Solvents (SPS) as their polarity can change between that of a molecular liquid and that of an ionic liquid, if triggered by the presence of CO2.
One other line of research in this general topic deals with interfacial tension dependence on metal extractant structure, diluent and acid concentration as a tool to reason structural and diluent effects on metal extraction profiles, namely iron from chloride media by fat amides and malonamides (in collaboration with Separation Science and Technology Group (SST) of CQB).
Some studies involving acoustic properties of biodiesels have also been carried out with CCMM and CICECO (Univ. Aveiro) providing important information on fuel injection features and NOx emissions.
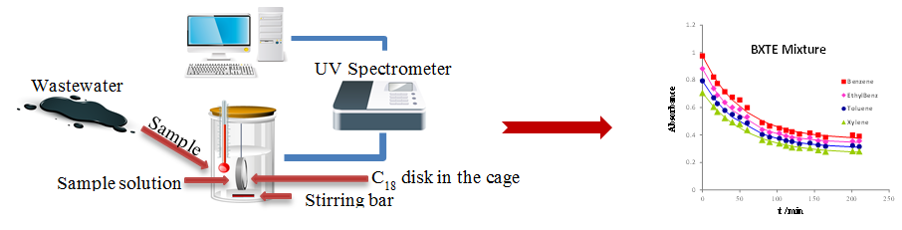
Testing and optimization of a solid phase extraction (SPE) procedure for the removal of BXTEs from wastewaters handled by the graphic industry through C18 disks, is also an example of a recent project developed with ISEC in the scope of this topic. The same methodology is now being applied for the removal of phenol derivatives (link).
Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal
Tel. (351) 217500000 | Fax. (351) 217500088
© Copyright - Structure and Reactivity Group